Hydrogen Production:
Electrolysis vs Gallium-Aluminum-Water Reaction
With the decline of fossil fuels and the need to develop reliable and sustainable energy sources, hydrogen is being considered as an alternative fuel that produces no CO2, nitrogen, and sulfur emissions.
There are, of course, different hydrogen production technologies. We will explore two of them: Standard Electrolysis and Gallium-Aluminum-Water Reaction
Standard Electrolysis
Standard Electrolysis is the process of splitting water into hydrogen and oxygen through the use of an electric current. While the technology was first developed in the 1920s, it has seen very little commercial use due to its historical high cost.
However, in the last decade has been significant improvements that have reduced their costs between 30 and 50%, depending on the technology.
Generally speaking, electrolysis is one of the best methods to produce hydrogen because it only uses water as produces only oxygen as a byproduct of the process. Besides, electrolysis uses DC energy in its processes, allowing for the use of renewable energy sources, like solar photovoltaic and wind energy.
At a 100% efficiency, an electrolysis process would be able to produce 0.03 kg H2/kWh of energy required. This value is scaled down by a conversion factor depending on the technology type as shown in Fig. 1.
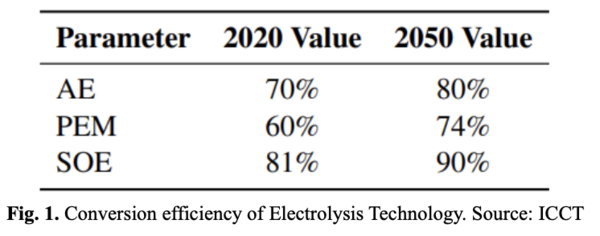
Still, it is a very expensive process. According to a study in the International Journal of Hydrogen Energy, the price of hydrogen obtained by electrolysis can range between 22 and 78 USD/GJ of energy generated. By comparison, this price is three times greater than the price obtained using steam reforming processes.

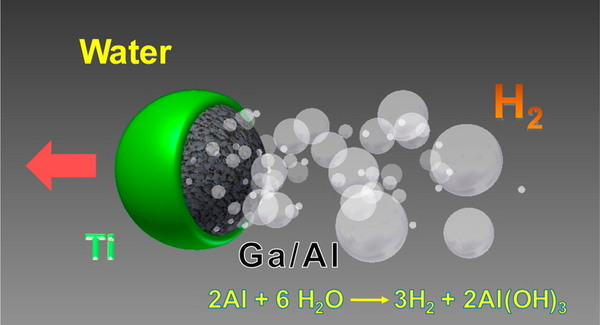
Another option to produce hydrogen comes from the reaction of water and specific metals like aluminum. In theory, the aluminum metal can react with water at room temperature and form hydrogen molecules with either an aluminum oxide or hydroxide as by products.
In practice, however, aluminum metal is coated with a naturally occurring layer of aluminum oxide, which makes it impossible for the reaction to occur. To use this reaction as a reliable source of hydrogen, it´s necessary to develop a practical mechanism that allows to continuously remove or disrupt the formation of the oxide layer.
Some research has determined that using molten aluminum allows, such as gallium-aluminum, can prevent the formation of a consistent oxide layer. It must be pointed out that the gallium used in this process can be easily recovered and used many times without reducing the system´s efficiency.
Some recent research has developed a mechanism to improve substantially hydrogen generation. This system allows for continuous and fast water splitting, allowing it to generate 130 mL of hydrogen per gram of gallium-aluminum alloy.
Nevertheless, there is no economically viable mechanism for its use yet.
Conclusions
As we have explored above current technology on hydrogen generation, particularly Electrolysis and Gallium-Aluminum Reaction with Water, are still being developed.
It remains to be seen if these processes can be scaled up to be practical and economically viable for large scale hydrogen production.
Contact info:
email: info@o-brien.tech
Phone: +852 9846 0670
www.o-brien.tech | www.windcycle.energy
